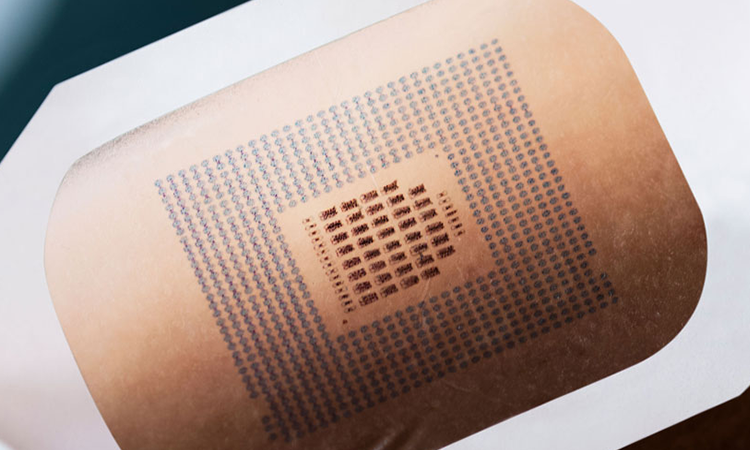
In the medical field, there are many diagnostic devices to measure biological information, including electrocardiogram, electromyogram and electroencephalogram, which involve electrodes that are attached to the human body. With the promotion of device miniaturization as a result of the advancement of IT and sensor technologies in recent years, and the simultaneous growing interest in preventive medicine, a wide range of wearable biosensors have emerged in the healthcare field. In the field of prevention and diagnosis, acquiring and monitoring biological information in daily life can be expected to contribute to the discovery of latent health risks such as the unexpected, sudden onset of arrhythmia. In the therapeutic field, wearable biosensors used in combination with surgical devices also contribute to the development of minimally invasive treatments based on the advancement of surgical techniques.
Challenges in the development of wearable biosensors
There are many design-related matters to consider when proceeding with the development of wearable biosensors.
What kind of structure is required to obtain stable signals with less noise? It is necessary to consider the usage environment (e.g., whether measurement is possible while patients are moving or if they have to be at rest), and then check the design items such as weight and size and their feasibility.
Among these, the effects on the human body may be raised as important matters to be checked. Wearable devices such as biosensors may be applied continuously for several hours to several days. Since they are used by being in contact with the human body for long hours, various matters regarding safety should be checked, e.g., the effects on the human body, etc.
For example, the most commonly used specifications are biocompatibility tests, i.e., checking whether an electrode or adhesive that is in contact with the skin causes a rash or inflammation of the skin.
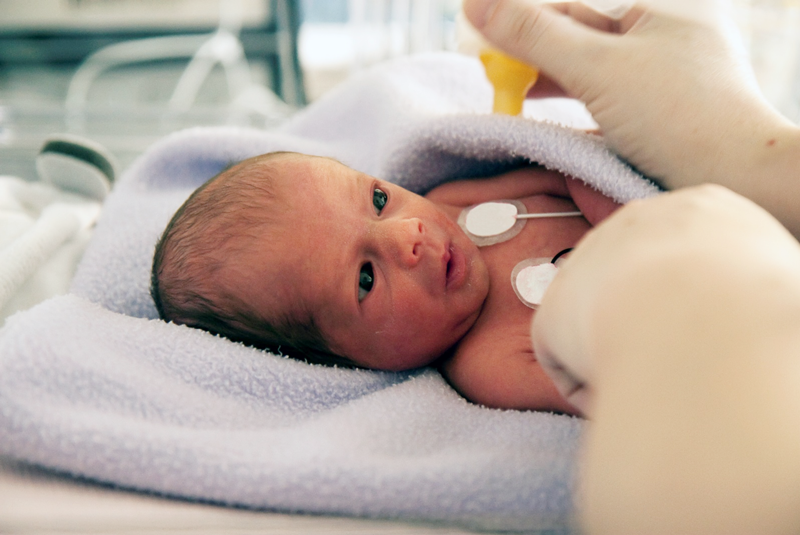
Another example is to check whether the adhesiveness of an electrode that is attached to the skin is sufficient so that it will not come off due to dry or sweating skin over a long period of time. Indicators of the adhesiveness to the skin and the burden on the skin should be appropriate, e.g., whether it is painful to remove the adhesive and whether patients develop redness or rash because the corneum of the skin is peeled.
There are large differences in skin between different individuals. For example, the condition of the skin differs between elderly people and newborn babies. Even in the same patient, the skin condition varies depending on the environment and season.
Concerning the characteristics of the skin burden that are difficult to evaluate, the methods of tests other than the general biocompatibility test have not been established in the medical industry. Therefore, companies that initiate development have to devise their own methods of design verification and validation taking these viewpoints into consideration, and they encounter challenges with design and development.
Nissha’s experience with and knowledge of the development of wearable devicessafety
With the development of wearable devices, it is not only necessary to consider the technologies involved in the design and manufacture to achieve certain functions and shapes that are safe and easy to use, but also to have the knowledge to select the optimal materials and usability, which may vary between individuals.
Nissha has a long history of contract design/manufacturing of medical devices such as wearables and biosensors. We have extensive experience and knowledge of the design, manufacture, evaluation and material selection of wearable devices.
Particularly concerning the assessment of materials used in wearable devices, we have established our own method in the course of assessing various materials of many manufacturers over many years.
For example, the adhesives of electrodes are quantitatively assessed for adhesiveness, which varies according to the condition of the skin with the cooperation of many subjects or by using artificial skin to measure the peel force.
The pain when peeling an adhesive from the skin is semi-quantitatively measured in combination with the sense of pain, which varies between different individuals, in comparison with a reference adhesive. The amount of removed corneum on the adhesive is assessed visually and numerically for physical irritation by peeling the adhesive off the skin. We also joined the Japanese Society for Cutaneous Health, and are constantly obtaining new information for development.
As a partner in the design/development of wearable devices
Please consult with Nissha about the manufacture/development of wearable devices. Nissha does not only have abundant experience with and knowledge of manufacturing technologies but also of materials and evaluations.